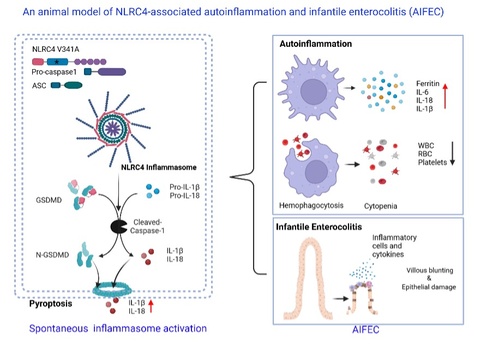
Gain-of-function mutations in the inflammasome sensor NLRC4 causes autoinflammation with infantile enterocolitis (AIFEC), a rare but often fatal monogenic disorder characterized by severe neonatal colitis, systemic hyperinflammation, and macrophage activation syndrome (MAS). A defining yet poorly understood feature of AIFEC is its striking age dependency: fulminant intestinal disease manifests in infancy but frequently resolves with age, while systemic autoinflammation persists. Progress in understanding disease mechanisms and testing targeted therapies has been limited by the lack of animal models that faithfully recapitulate the human phenotype.
From Zizhen Kang, PhD and Yuhang Wang, PhD, this study reports the generation of a conditional NLRC4 V341A knock-in mouse that robustly mirrors the clinical and immunopathological hallmarks of AIFEC. Germline activation of NLRC4 V341A triggered spontaneous inflammasome hyperactivation, evidenced by caspase-1 and gasdermin D cleavage and excessive IL-1β and IL-18 production. Neonatal mice rapidly developed severe infantile enterocolitis, with villus blunting, epithelial pyroptosis, loss of goblet and Paneth cells, disruption of tight junction proteins, compromised barrier integrity, profound diarrhea, and failure to thrive. In parallel, these pups exhibited systemic autoinflammation resembling MAS, including cytopenia, hemophagocytosis, hyperferritinemia, multiorgan injury, and early lethality.
A key insight from this model is that disease arises postnatally rather than in utero. NLRC4 V341A mice are born without overt pathology but develop rapid intestinal and systemic inflammation within days of birth, coinciding with epithelial maturation, microbial colonization, and heightened metabolic demand. This temporal window provides a mechanistic explanation for the infancy-restricted presentation of AIFEC in humans and highlights neonatal life as a uniquely vulnerable period for inflammasome-driven pathology.
Importantly, this model enabled systematic preclinical testing of therapeutic strategies. IL-18 blockade with recombinant IL-18 binding protein significantly improved survival, attenuated colitis, and reduced systemic inflammation, establishing IL-18 as a central disease driver. TNF neutralization proved even more effective, nearly normalizing survival and markedly suppressing both intestinal and systemic pathology, identifying TNF signaling as a critical and clinically actionable pathway in AIFEC. Unexpectedly, the authors uncovered severe hypoglycemia as a consistent feature of neonatal NLRC4 activation. Remarkably, glucose supplementation alone partially rescued survival, restored epithelial integrity, and mitigated systemic inflammation, revealing metabolic insufficiency as an important amplifier of autoinflammatory disease.
The full study has been published and highlighted in Cellular & Molecular Immunology. This work was supported by startup funding from the Department of Pathology and involved collaboration with other laboratories as described in the paper. Read the full study (PMID: 41116055) and highlights (PMID: 41339480) here.